What Is the Most Likely Oxidation State of Nitrogen
Oxidation state 0 which is found for all elements is implied by the column with the elements symbol. Explaining what oxidation states oxidation numbers are.
What Is The Formula To Find Maximum And Minimum Oxidation Number Of An Element Quora
How to calculate Oxidation Number.
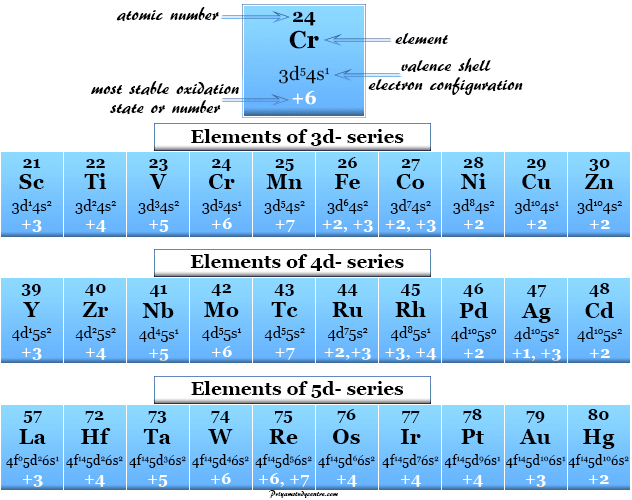
. The above table can be used to conclude that boron a Group III element will typically have an oxidation state of 3 and nitrogen a group V element an oxidation state of -3. The most common oxidation state of nitrogen is zero. Typical oxidation states of the most common elements by group Transition metals are not included as they tend to exhibit a variety of oxidation states.
-2 The correct answer is Option C. 4 NO2 Nitrogen dioxide a brown gas usually produced by the reaction of concentrated nitric acid with many metals. In a chemical reaction if there is an increase in oxidation state then it is known as oxidation whereas if there is a decrease in oxidation state it is known as reduction.
Oxidation states simplify the whole process of working out what is being oxidised and what is being reduced in redox reactions. A As the oxidation number on the metal increases the valence-state electronegativity increases and the oxides change from acidic to basic. It is reduced to nitrous acid HNO 2 in which the OS of nitrogen is 3 and oxidized to nitric acid HNO 3.
Valency is a pure number whereas oxidation number has positive or negative value. The Earths atmosphere is 78 N2 - elemental nitrogen and there is a lot of it in the atmosphere making it the most common form in which we find nitrogen. Oxidation states of nitrogen Ox.
The format of the table based on one devised by Mendeleev in 1889 highlights some of the periodic trends. Oxidation number and valency are having fixed value. Since the net charge on the anion is -1 the nitrogen atom must have a positive oxidation state of 5.
Oxidation occurs when the oxidation number of an atom becomes larger. Is oxidation state and oxidation number same. While fully ionic bonds are not found in nature many bonds exhibit strong ionicity making.
In sodium compounds sodium only forms 1 oxidation number. It was -3 Omg Im so sorry I didnt see -3 on here lol. In an atom of an element has one or two valence electrons and is not a helium atom the most likely oxidation state.
What is the most likely oxidation state of nitrogen. The oxidation state or oxidation number is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionicIt describes the degree of oxidation loss of electrons of an atom in a chemical compoundConceptually the oxidation state may be positive negative or zero. The most common oxidation states are in bold.
The oxidation states are also maintained in articles of the elements of course and systematically in the table Infobox elementsymbol-to-oxidation-state An overview is here. The oxidation state sometimes referred to as oxidation number describes the degree of oxidation loss of electrons of an atom in a chemical compound. The lowest known oxidation state is 4 for carbon in CH 4 methane.
What is the most likely oxidation state of nitrogen - 18973552 bigfoot42069 bigfoot42069 11052020 Chemistry College answered What is the most likely oxidation state of nitrogen 2 See answers Advertisement. Oxidation states are straightforward to work out and to use but it is quite difficult to define what they are in any quick way. State Species 5 NO3-Nitrate ion oxidizing agent in acidic solution.
C As the oxidation number on the metal increases the valence-state. The manganese cation outside is being combined with 2 nitrate anions in the formula unit. Nitrogen dioxide NO 2 reacts with water to give nitric and nitrous acids Ostwald process.
It is a disproportionation reaction. Typical oxidation states of the most common elements by group. Oxidation States of Nitrogen Oxidation HNO3 N2O 1 5 -3 NO 2 NH3-13 HN3 Reduction.
Oxidation and reduction are therefore best defined as follows. The oxidation state of carbon increases from 2 to 4 while the oxidation state of the hydrogen decreases from 1 to 0. The highest known oxidation state is 9 in the tetroxoiridium IX.
This table is based on Greenwoods1 with all additions noted. How is an elements most likely oxidation state related to its valence electron. Therefore the charge on the manganese cation is 2 and so is its oxidation state.
B As the oxidation number on the metal increases the valence-state electronegativity increases and the oxides change from basic to acidic. It dimerizes to form N2O4. The Nitrogen in NO 2 is in 4 oxidation state.
Can be either an. 3 Pls dont steal points youll be reported 2 See answers sam2322 sam2322 Im pretty sure its A. What is the most likely oxidation state of nitrogenA.
The above table can be used to conclude that boron a Group III element will typically have an oxidation state of 3 and nitrogen a group V element an oxidation state of -3. Maintenance improvements edit. Oxidation state is defined as the number which is given to an atom when it looses or gains electron.
In the elemental state the oxidation state is zero. But some types of atoms such as. Correct answer to the question What is the most likely oxidation state of nitrogen.
What is the oxidation state of each element in MN no3 2. 3 NO2-Nitrite ion in basic solution and nitrous acid in acidic solution. Transition metals are not included as they tend to exhibit a variety of oxidation states.
Reduction occurs when the oxidation number of an atom becomes smaller.

19 1 Definition Of Oxidation State For Carbon Organic Chemistry Ii

Lithium Definition Properties Use Facts Britannica

19 1 Definition Of Oxidation State For Carbon Organic Chemistry Ii
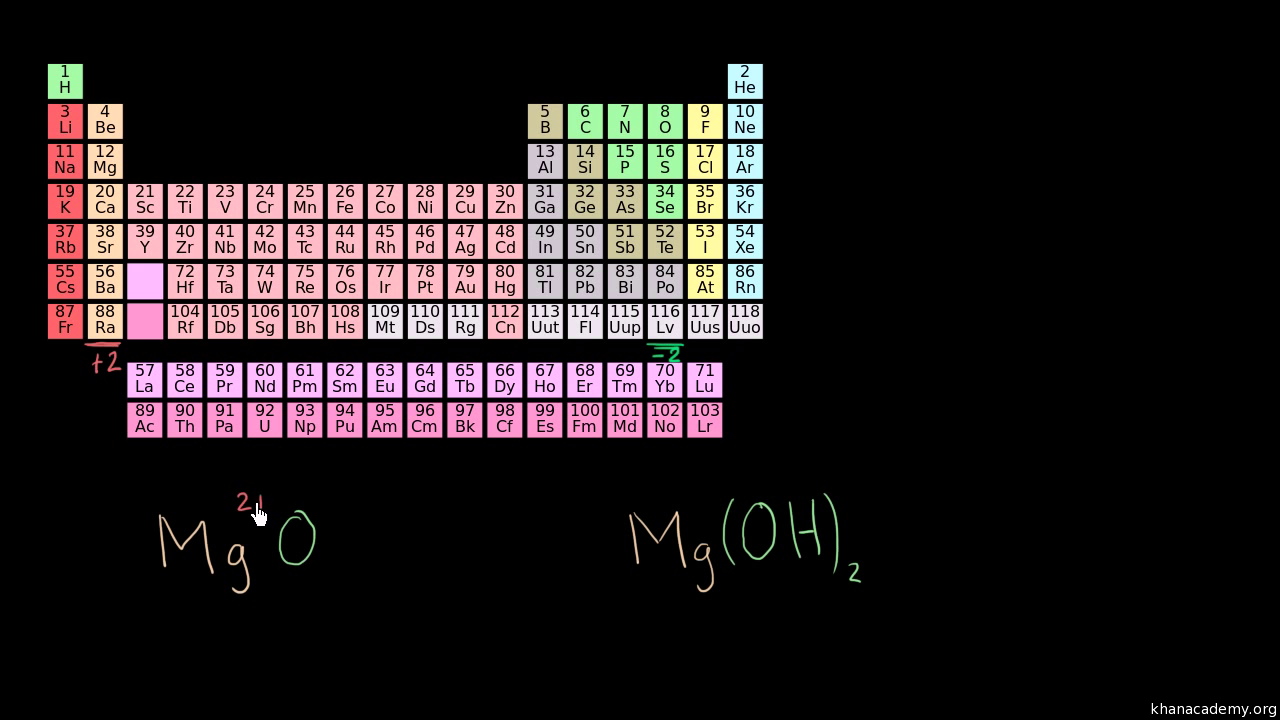
Practice Determining Oxidation States Video Khan Academy

Lesson Video Oxidation Numbers Nagwa

How To Find Oxidation Numbers Rules And Examples Youtube

How To Find Oxidation Numbers Rules And Examples Youtube

Oxidation States Introduction To Chemistry
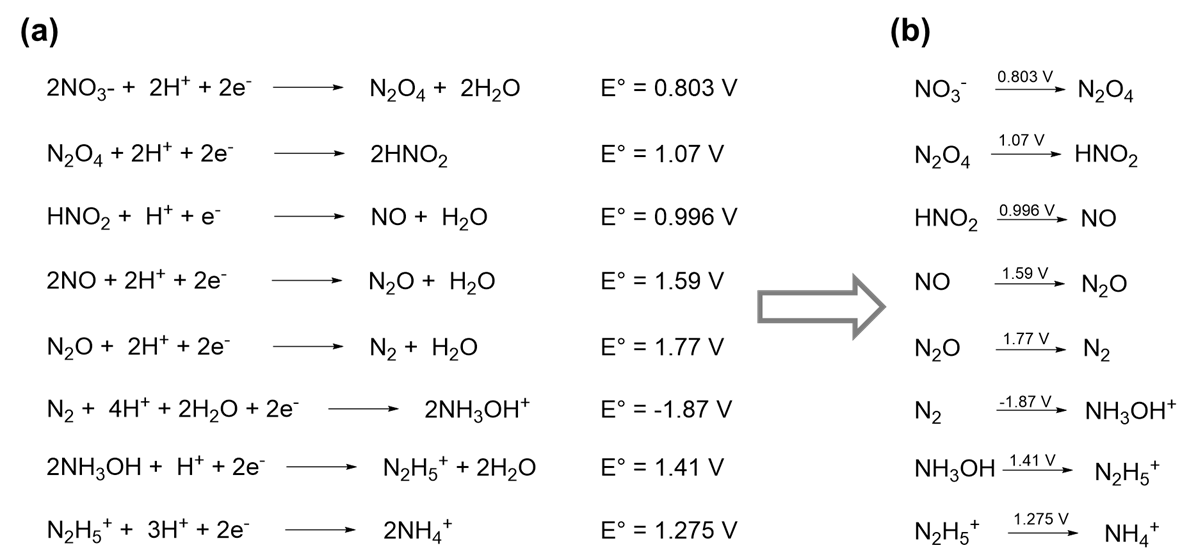
8 1 4 1 Latimer Diagrams Summarize Elements Redox Properties On A Single Line Chemistry Libretexts

4 3 Formal Charge And Oxidation State Chemistry Libretexts
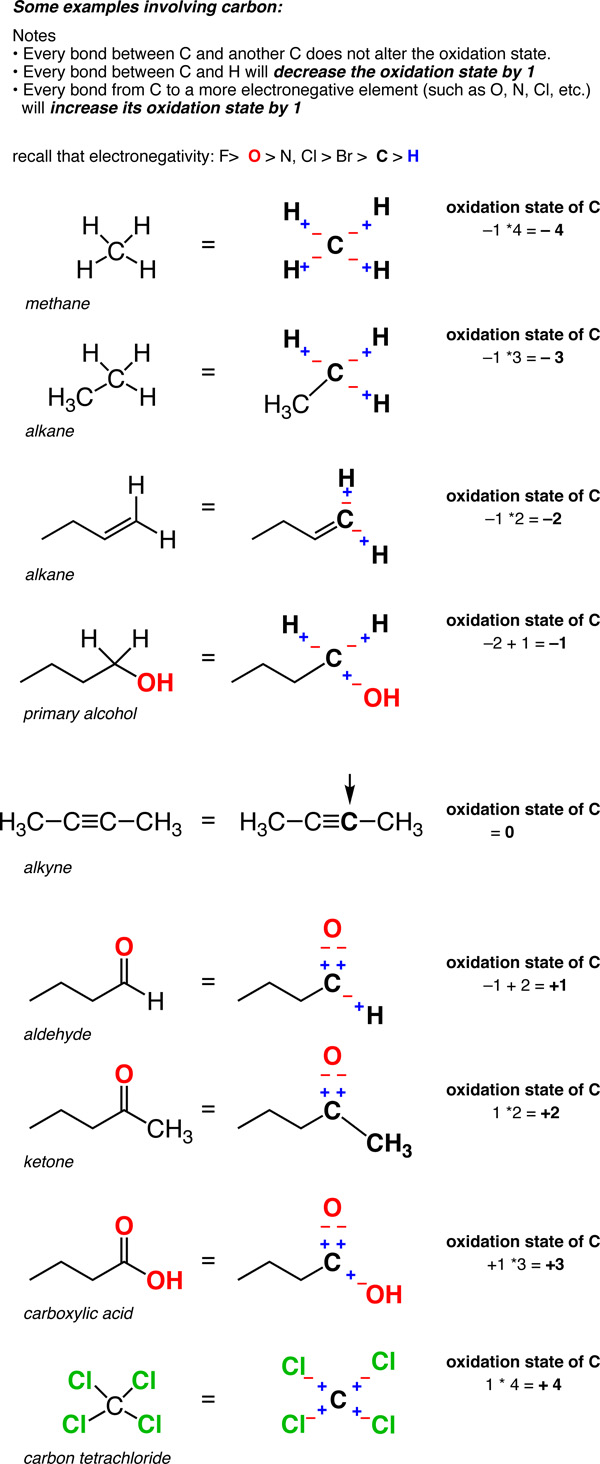
19 1 Definition Of Oxidation State For Carbon Organic Chemistry Ii
Why Do Some Elements Have More Than One Oxidation State Quora
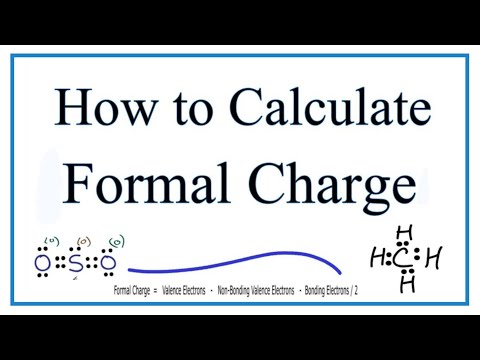
4 3 Formal Charge And Oxidation State Chemistry Libretexts

Practice Determining Oxidation States Video Khan Academy

What Is The Most Common Oxidation State Of Nitrogen Quora

Oxidation Number Periodic Table Elements Definition Rules
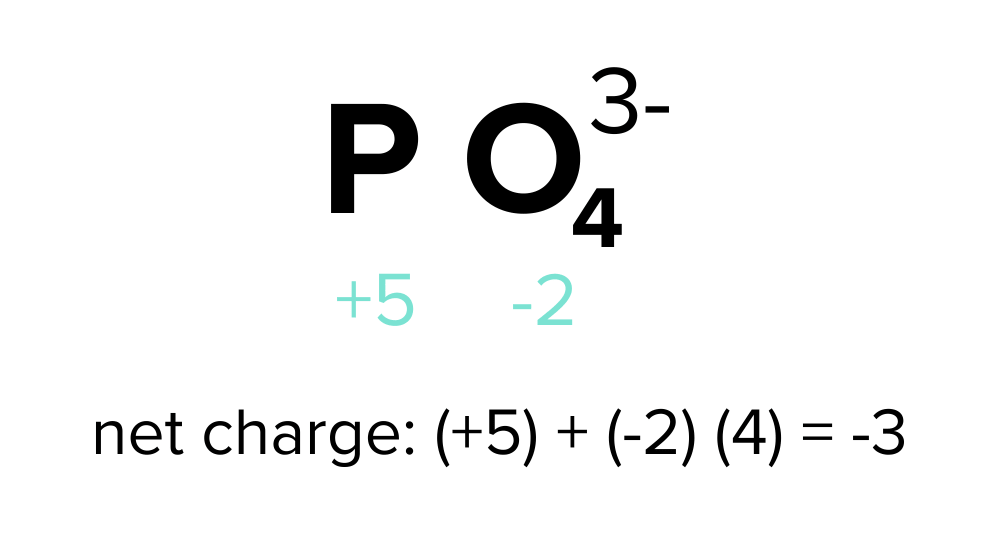
Oxidation And Reduction Reactions For The Mcat Everything You Need To Know Shemmassian Academic Consulting
Comments
Post a Comment